EVOLUTION OF COGNITIVE IMPAIRMENT IN DEPRESSED ELDERLY PATIENTS
Abstract
Background: Depression in elderly is generally undiagnosed and undertreated, many still considering its symptoms as part of normal aging process. Some studies suggest that depression is a risk factor for dementia. In the same time cognitive impairment, often considered as dementia prodrome, is an important symptom of depression in elderly. Objectives: In this research we tried to evaluate cognitive impairment and depression symptoms in elderly patients using three important rating scales: Mini-Mental State Evaluation (MMSE), Geriatric Depression Scale (GDS) and Hamilton Depression Rating Scale (HDRS). Apart from clinical features we assessed a number of risk factors for depression symptoms like gender and medical history. Material and methods: The research included 37 depressed patients admitted in the ward of Clinical Psychiatric Hospital ”Alexandru Obregia” Bucharest, during one year. The subjects were female and male patients and the age ranged from 66 to 90 years old. All patients were evaluated using 3 scales: MMSE, GDS and HDRS. These scales were applied at inclusion and after 6 weeks of treatment. Results: Most of the patients in this study were females (73%). The average educational level in this group was of 8 years of study. MMSE scores increased after 6 weeks of treatment implying improvement of cognitive impairment. The depressive clinical symptoms were evaluated using GDS and HDRS. Improvement of depressive symptoms of entire group presented was observed through increased scores after 6 weeks of treatment. Conclusions: Cognitive impairment is an important aspect of depressive symptoms that should be properly evaluated, using different scales, for a more precise diagnosis.
INTRODUCTION
Depression in elderly is frequently undiagnosed, many considering these symptoms as part of the normal aging process. It is considered that up to 80% of elderly people that suffer from different depression symptoms will not receive proper treatment (1). This is probably due to the heterogeneous clinical presentation that sometimes can strongly differ from that at younger ages (2).
Today it is generally accepted that almost 15% of the elderly population suffers from a type of depression (2).
One study in Great Britain that applied the Geriatric Mental Scale on a large population of persons over 65 years showed that 2-4% of the questioned population suffered from major depressive disorder, whereas the prevalence of minor depressive disorder was 11% for women and 5% for men (3).
Depression in elderly subjects is part of a complex physical and mental constellation that could gather a large range of conditions. In some cases depressive symptoms can explain the poor adherence to treatment that in the end leads to higher morbidity and mortality risks (4). For example combined with cardiac disease it can precipitate a cardiac episode and influencing the recovery (5).
An important aspect of depression in elderly that sometimes is overlooked is the suicidal risk. Some studies have shown that the suicidal rate in depressed patients with age 80 to 84 years old is twice that of the general population (6, 7).
Due to the important implications of patient’s evolution many studies have tried to identify a number of risk factors that can prone elderly to develop depression. It has been shown that female gender and lack of social and familial support can precipitate depressive episodes (8). Also different stressful events in life, including history of medical disorders and smoking can play a major role in onset of depression in elderly (9, 10).
The heterogeneity of depression in elderly can be observed also in the clinical features. Most frequently patients present generalized anxiety and irritability (3). Apart from these a lot of somatic complaints are be present.
An important and specific characteristic of depression at elderly patients is the cognitive and functional impairment (9). The cognitive impairment is an important problem in elderly depressed patients, mainly because cognitive deficits can be observed in many neurologic and psychiatric disorders. Even if the deficit is important, significant cortical dysfunction like aphrasia or apraxia are rare, while clinical dementia seldom appears (11, 12). Studies show a correlation between depression and brain lesions. It is difficult to distinguish mild lesions that sometimes are considered as part of the natural aging process and those that are significant (13). O’Brien et al (14) observed, after studying a group of elderly subjects using magnetic resonance imaging (MRI) that white mater lesions are significantly more common at depressed patients than in the control group. These white matter brain lesions can be a reason for poor response to initial treatment (15, 16). Unfortunately these conclusions are difficult to generalize because only severe lesions have been correlated with depression, while mild to moderate lesions are difficult to distinguish from what is considered normal aging process.
Simpson et al (17) emphasized that there should be a specific combination of white matter lesions in order to consider MRI relevant in diagnosing and predicting the outcome of depressed elderly patients. These lesions should be found in basal ganglia, pons and frontal lobe (17). This conclusion is supported by many other studies (18, 19).
Treating depression at old age can be challenging even for the experienced clinicians. Despite this setback it has been shown that antidepressant treatment has a better influence on the outcome compared with no treatment (20). Newer antidepressant therapies are a good choice for elderly patients mainly because they have better tolerability, safety and are easy to dose (21). The duration of treatment might be longer than in younger patients, at least 2 years after the episode remission or it may become a permanent therapy.
OBJECTIVES
This study tries to evaluate in what degree cognitive impairment at admission is a temporary state part of depressive symptoms or if depression is just one phase, and cognitive impairment in fact announces a future dementia. In order to evaluate the clinical status we assessed a number of risk factors that can influence the onset of depression in elderly: gender, level of education and medical history.
We evaluated patients from two different perspectives: first from the cognitive impairement point of view using Mini-Mental State Evaluation (MMSE) and second from the depression point of view using two different scales: Geriatric Depression Scale (GDS) and Hamilton Depression Rating Scale (HDRS).
METHODS
The prospective study included 37 patients admitted in a ward of Clinical Psychiatric Hospital ”Alexandru Obregia”, Bucharest over one year, for depressive symptomatology. The subjects were female and male patients and the age ranged from 66 to 90 years old.
All patients were evaluated using 3 scales: MMSE, GDS and HDRS. MMSE is a frequently used scale to evaluate cognitive impairment in dementia. GDS is generally used in geriatric facilities to evaluate depression in elderly. Because answers are ”yes” and ”no”, compared to the normal 5 response set, it is easier to use on elderly patients. The scale has two versions, the original one with 30 questions and a shorter version with 15 questions, the one we used. HDRS is a well known and vastly used scale to evaluate depression symptoms and treatment efficacy.
RESULTS
The study included 37 patients from which 27 were female (73%) and 10 were men (27%). This distribution is considered to be normal because female gender is a risk factor for depression in elderly.
According to age patients were divided in 5 groups. Most of the patients were in the 71-75 years group (29, 73%), followed by the 66-70 years group (24, 32%), the last being the 86-90 years group (10, 81%) (). It has been shown that depression is more frequent in patients over 70 years old (22) compared to those less than 70 years old. The decrease of number of depressed patients over 75 can be considered normal because the mortality rate increases due to complex somatic features that these patients have.
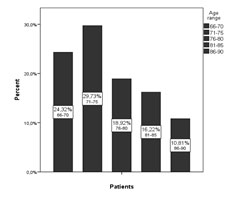
Figure . Distribution of patients according to age
The education level was also divided in subgroups according to the number of study years. Most of them had up to 8 years of education (70, 28%) while university education was the least frequent in this group (10, 81%). Most of the patients presented medical history (63%) because in general elderly patients have a variety of medical conditions that accompany depressive symptoms. Cognitive impairment was measured using MMSE. In our study group MMSE scores were influenced by the years of education. Most of the patients with 4 years of education scored 17 to 19 points on MMSE, whereas patients with more than 5 years of education scored 20 points or more. These results can be consistent with the supposition that MMSE scores are influenced considerably by the education level of patients (23), in some cases cognitive impairment being concealed by knowledge level.
Medical history has an important role in onset and evolution of depression in elderly. Due to the fact that cognitive impairment is also an important part of depression in elderly we tried to see if, in our group of patients, the medical history can influence the degree of cognitive impairment. More than 60% of the patients with cognitive impairment had medical history. In the same time we observed that the percentage of patients with cognitive impairment increases as the debut of depression symptoms is later in life. Almost 80% of patients with late onset of depression symptoms have mild cognitive impairment.
There is an important difference between the MMSE scores before the treatment and after 6 weeks of antidepressant treatment. Before the treatment, patients with medical history (MH) had overall higher MMSE scores compared to patients without medical history (2). In mild cognitive impairment subgroup the difference between MMSE scores at patients with or without medical history is smaller compared to the difference in subgroup with moderate cognitive impairment. Also in the subgroup with moderate cognitive impairment patients with medical history accomplished higher MMSE scores than those with no medical history.
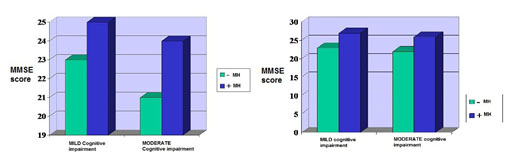
Figure 2. MMSE before and after 6 weeks of treatment
The difference observed before treatment tends to decrease after 6 weeks of treatment (2). MMSE scores become almost the same in the mild and moderate cognitive impairment subgroups. The largest difference between MMSE scores before and after 6 weeks of treatment is observed at patients with no medical history. We can conclude that cognitive impairment, especially in patients with no medical history, is more probably part of depression. This is likely the reason why after 6 weeks of adequate treatment MMSE scores increased.
Depressive symptoms have been evaluated using Geriatric Depression Scale (GDS). On this group of patients we used the shorter version with 15 questions. According to their scores patients were divided in two groups: 6-9 points and 9-14 points (Figure 3). Before treatment most of the patients were in the 6-9 points group.
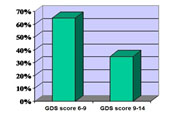
Figure 3. GDS scores at inclusion
The majority of patients with medical history were in the
9-14 points group, compared to the group with 6-9 points which included most of the patients with no medical history.
After 6 weeks of treatment in the subgroup of patients with no medical history we observed a reduction of GDS score. The decrease of score was observed at patients with mild cognitive impairment and at those with moderate cognitive impairment. The evolution according to GDS score in patients with medical history was similar to that in those with no medical history. Before treatment all patients with medical history had higher GDS scores compared to scores after 6 weeks of treatment. The scores decreased in the subgroup of patients with mild cognitive impairment but also at those in the subgroup with moderate cognitive impairment.
We can conclude that medical history can influence GDS scores at admission. In the same time the evolution was favourable, and GDS scores decreased after 6 weeks of treatment as the depressive symptoms improved.
The second scale used to evaluate depression in elderly was HDRS. It is considered the gold standard for evaluating depression. Most of the patients in this study had mild depression according to HDRS scores (Figure 4). According to HDRS scores most of the patients with moderate depression had no medical history, while most of the patients with mild depression had medical history.This result is consistent with the consideration that somatic medical history can influence depression. Patients with no medical history are more likely to present only depressive symptoms and so to have a higher HDRS score.
At 6 weeks of treatment we observed a reduction of HDRS scores in the subgroup without medical history. Similar evolution had HDRS scores for the subgroup of patients with medical history after 6 weeks of proper treatment. Overall HDRS scores for patients with medical history were smaller than those for patients without medical history.
CONCLUSIONS
Depression in elderly is an important medical issue that should not be overlooked. It can influence the entire evolution of a patient. A significant part of depression in elderly is cognitive impairment. It is difficult to definitely say whether cognitive impairment in depressed elderly patients is part of depression with late onset or this clinical feature is the debut of dementia.
This conclusion is supported by Austin et al (11) who observed that cognitive impairment is more severe in elderly patients but is not seen exclusively in this category of patients. Even if the deficit is important, cortical dysfunction like aphasia or apraxia are rare in depression. Also Abas et al (12) underlines that 70% of depressed patients with average age of 70 years old present important cognitive impairment, but not a clinical dementia. In the latter study as well, it has been shown that 1/3 of elderly subjects continued to present cognitive impairment despite proper and effective depression treatment (12).
For a better evaluation of elderly patients that present such symptoms there should be used more scales for accurate results. In our study group MMSE scores decreased after 6 weeks of treatment leading to the conclusion that cognitive impairment in this case is part of depressive symptomatology. Studies have demonstrated that the level of education can influence MMSE results (23). O’Connor et al (24) showed in a study on a community population that almost 2% of the interviewers that scored 26 point or more on the MMSE evaluation had important cognitive impairment. He observed that this result was due to educational level.
The conclusion that cognitive impairment is part of depression is supported by the severity of depression, as illustrated by the scores on GDS and HDRS. The fact that GDS scores were similar to HDRS scores in our study supports the literature conclusion that GDS results are comparable to those of HDRS. Weintraub et al (25) showed that GDS is a reliable scale that can differ depressed from undepressed patients and its results are comparable to those of HDRS.
The most visible improvement of cognitive impairment and depressive symptoms, after 6 weeks of treatment, was observed in the subgroup of patients without medical history mainly because depressive symptomatology is their only medical problem. In contrast patients with medical history did not have such a good evolution of scores mainly because their depressive symptoms were part of a more complex symptomatology.
REFERENCES
1. Consensus Development Conference. Diagnosis and treatment of depression of late life. JAMA 1992;268: 1018-1029.
2.Lovestone S, Howard R. Depression in Elderly People. London
Martin Dunitz Ltd, 1996, 3-4.
3.Cornelius LE. Depression in Old Age. West Sussex: John Wiley and
Sons Ltd., 1994, 2-4.
4.Carney RM, Freedland KE, Eisen SA, Rich MW, Jaffe AS. Major depression and medication adherence in elderly patients with coronary
artery disease. Health Psychology 1995;14: 88-90.
5.Goldberg J. Depression in the Elderly. WebMed 2012.
6.Conwell Y, Brent D. Suicide and aging. International Psychogeriatrics
1995;7(2): 149-64.
7.Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. Web-based Injury Statistics Query and Reporting System (WISQARS).
8.Cole MG, Dendukuri N. Risk Factors for Depression Among Elderly
Community Subjects: A Systematic Review and Meta-Analysis. Am J Psychiatry 2003;160: 1147-1156.
9.Weyerer S, Eifflaender-Gorfer S, Köhler L et al. Prevalence and risk factors for depression in non-demented primary care attenders aged 75 years and older. J Affect Disord 2008;111(2-3): 153-63.
10.Osborn DP, Fletcher AE, Smeeth L et al. Factors associated with depression in a representative sample of 14 217 people aged 75 and over in the United Kingdom: results from the MRC trial of assessment and management of older people in the community. Int J Geriatr Psychiatry
2003;18(7): 623-30.
11.Austin MP, Ross M, Murray C, RE, Ebmeier KP, Goodwin GM. Cognitive function in major depression. Journal of affective disorders
1992;25: 21–29.
12.Abas M, Barbara J, Levy S, Levy R. Neuropsychological deficits and
CT scan changes in elderly depressives. Psychological Medicine
1990;20: 507-520.
13.O’Brien J, Ames D, Chiu E, Schweitzer I, Dermond P, Tress B. Severe deep white matter lesions and outcome in elderly patients with major depressive disorder: follow up study. BMJ 1998;317: 982.
14.O’Brien J, Desmond P, Ames D, Schweitzer I, Harrigan S, Tress B. A magnetic resonance imaging study of white matter lesions in depression and Alzheimer’s disease. Br J Psychiatry 1996;168: 477–485.
15.Hickie I, Scott E, Mitchell P, Wilhelm K, Austin MP, Bennett B. Subcortical hyperintensities on magnetic resonance imaging: clinical correlates and prognostic significance in patients with severe depression.. Biol Psychiatry 1995;37(3): 151-60.
16.Matsubayashi K, Shimada K, Kawamoto A, Ozawa T. Incidental brain lesions on magnetic resonance imaging and neurobehavioral functions in the apparently healthy elderly. Stroke 1992;23: 175-180.
17.Simpson SW, Jackson A, Baldwin RC. Subcortical hyperintensities i n l a t e – l i f e d e p r e s s i o n : a c u t e r e s p o n s e t o t r e a t m e n t a n d neuropsychological impairment. Int Psychogeriatr 1997;9(3): 257-75.
18.Rabins PV, Pearlson GD, Aylward E, Kulmar AJ, Dowell K. Cortical magnetic resonance imaging changes in elderly inpatients with major depression. Am J Psychiatry 1991;148: 617-620.
19.Ranga K, Krishnan R. Neuroanatomic Substrates of Depression in the
Elderly. J Geriatr Psychiatry Neurol 1993;6: 39-58.
20.Scogin F, McElreath L. Efficacy of psychosocial treatments for geriatric depression: A quantitative review. Journal of Consulting and
Clinical Psychology 1994;62: 69-74.
21.Montano CB. Primary care issues related to the treatment of depression in elderly patients University of Connecticut Medical School, Farmington, USA. The Journal of Clinical Psychiatry 1999;60:
45-51.
22.D, Okifuji A, Scharff L. Chronic pain and depression: role of perceived impact and perceived control in different age cohorts. Pain
1995;61: 93–101.
23.Ylikoski R, Erkinjuntti T, Sulkava R et al. Correction for age, education and other demographic variables in the use of the Mini Mental State Examination in Finland. Acta Neurologica Scandinavica 1992;85:
391–396.
24.O’Connor DW, Pollitt PA, Treasure FP. The influence of education and social class on the diagnosis of dementia in a community population. Psychological Medicine 1991;21: 219-224.
25.Weintraub D, Oehlberg K, Katz I, Stern. Test Characteristics of the
15-Item Geriatric Depression Scale and Hamilton Depression Rating
Scale in Parkinson Disease. Am J Geriatr Psychiatry 2006;14(2):
169–175.
***



